Do I Need Calcium Chloride to Make Cheese
Many of the questions we get from cheese makers involve issues related to weak curds: the whey is not clear and the curds are too soft. We recommend that you send our technical advisor, Jim Wallace the particulars (jim@cheesemaking.com), but very often, the problem is caused by insufficient calcium.
The beauty of calcium chloride is that it adds calcium ions to the milk and slightly raises the amount of acidity during the cheese making process (more about this below). And, because it strengthens the protein molecules in the milk, it boosts the yield of our cheese by as much as 2-3%.
Most home cheese makers hedge their bets by using it. In fact, it's safe to say that almost all artisan and commercial cheese makers add it to their milk before renneting.
What is calcium chloride?
Food grade calcium chloride is nothing more than a natural mineral salt, used in pickles (pickling salt), beer, tofu, bottled water, soda, etc. According to Wikipedia, it is even used to slow the freezing of the caramel in caramel-filled chocolate bars. The FDA approves it as being a harmless substance.
Allergy information in our catalog about calcium chloride (click here)
Why do we use it?
By the time we're ready to turn our milk into cheese, it has been through a lot of changes. These impact the coagulation process and determine whether our curds will be as strong as we need them to be. (Even if we are using milk straight from the cow, sheep or goat, there are still factors which effect the milk- what the animal ate, what stage of lactation the animal is in, etc.) Whether our milk is raw or store-bought, the odds are great that it will benefit from the addition of calcium chloride.
Factors affecting our milk include:
- Types and breeds of the animals – Some milk is just naturally low in protein and calcium. Goat's milk, for example, is lower than cow's milk.
- Diet of the animals – The milk may be low in calcium if the animals have been eating young grass in the spring (which is low in minerals).
- Presence of infections – Udder infections cause an increase in somatic cells (to fight off the infection) and this introduces enzymes which attack the proteins and the calcium.
- Stage of lactation cycle – These same enzymes also increase in volume when the animal is at the end of it's lactation cycle and during the winter months.
- Heat – Pasteurization (heat) erodes the calcium in the milk, weakening the walls of protein. (In the picture below, you can see the difference in the shape of the proteins when they have been heated.)
- Cold storage – Even raw milk loses calcium in 24 hours of storage. (Most of the time, however, the proteins can be restored, at least partially, when the milk is heated for cheese making.)
How does it work?
It's all about the acidity of the milk. When milk comes from the animal, it is usually at a certain level (although this itself may be lower than usual if the animal is in the late stage of it's lactation cycle or if it's fighting off an infection). The goal of the cheese maker is to raise the acidity of the milk to the optimum point for coagulation. We use several tools to achieve this- usually heat, acids (lemon juice or vinegar), bacteria (starter cultures)* and enzymes (rennet). We use these tools in all kinds of different ways, combinations and speeds to achieve a wide variety of cheeses.
The goal is to separate the curds from the whey, but there is a complicated chemical process involved in this and a lot of it depends on the amount of calcium ions holding the protein molecules together. The amount of calcium ions has a lot to do with the acidity of the milk. If the acidity is too low, the milk does not have enough calcium ions to facilitate coagulation.
The best whey to explain this is for you to picture a wall of bricks. The bricks themselves are the protein molecules in the milk. The mortar is the calcium. The calcium holds the bricks together. During the course of making cheese, lactic acid tries to break up the protein molecules. Calcium isn't effected by the acid, so it creates a buffer for the protein "bricks." If there isn't enough calcium, there isn't enough "mortar" to hold the bricks together. The bricks get weak and the curds become soft. (This analogy comes from Gianaclis Caldwell's Mastering Artisan Cheesemaking.)
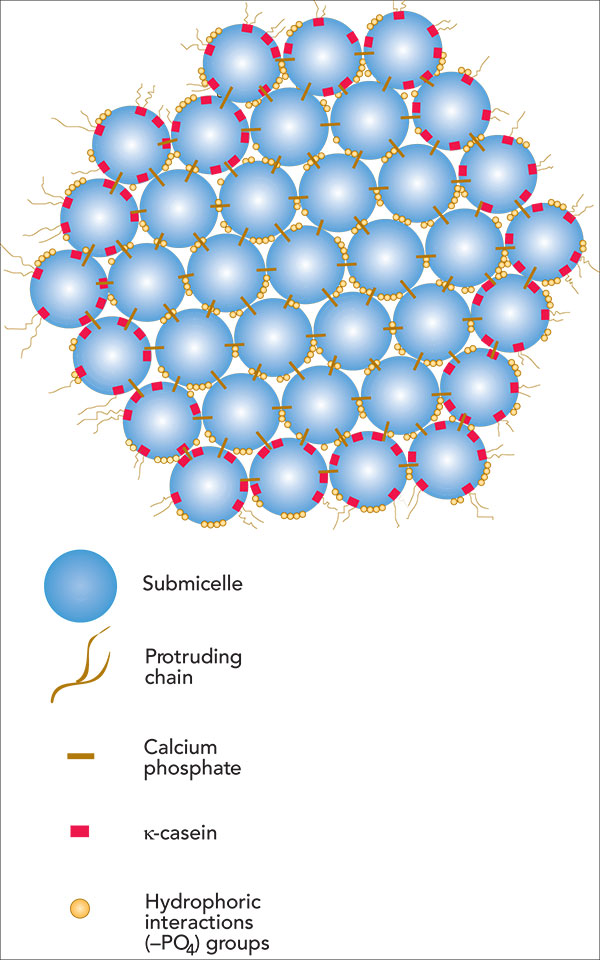
The submicelle is the protein and the lines holding them together are the calcium ions. This is one of 3 different ways of looking at the situation, shown in dairyprocesinghandbook.com
Can we measure this?
We can actually measure the amount of calcium ions in our milk with a meter, but this would be whey too expensive for home cheese makers. We are more interested in measuring the acidity at various stages in the process with an acid meter (E70).
How do we use it?
In our milk:
We add it to our milk at least 5 minutes before we add the rennet. Usually the amount is the same as the amount of (single strength) rennet we will be using. This is generally 1/8 – 1/4 teaspoon per gallon of milk (recommendations vary). We suggest that if you are using store-bought pasteurized and homogenized milk, you use up to 1/2 teaspoon per gallon (our FAQ section). (Note- no amount of calcium chloride will compensate for ultra-pasteurized milk.)
Please do not think that using more will be better- it will not. In fact, it will have a reverse effect and slow the coagulation of your curds.
Before you add it to your milk, dilute it in 1/8 – 1/4 cup of water (per gallon of milk used).
An example of how to use it: If you have added the culture to your milk and you are letting it ripen for one hour, add the calcium chloride solution after that and let the milk set for 5 more minutes before you add your rennet.
Food grade calcium chloride has already been diluted to a 32% solution. If you are making your own, from crystals, use distilled water.
In our brine:
Basically, we add it to our brine to create a balance in minerals between the cheese and the brine.
We recommend in our website's (How-To section) adding 1 tablespoon per gallon of brine when you first make it. Recommendations vary and Gianaclis Caldwell recommends a little more – 1 teaspoon per quart (Mastering Basic Cheesemaking). After we have made our brine, we do not have to add more calcium chloride as time goes by because the cheese will release enough calcium into the brine to maintain an equilibrium. (Adding whey to the brine will also help to keep the calcium level up.)
Where do we find it?
We sell it (click here) and most other cheese making suppliers carry it.
Beer and wine making supplies sell it.
You can use pickle crisp if you want to dilute it.

Formula for diluting calcium crystals (pickling salt), courtesy of Ian Treuer's great blog- Much To Do About Cheese
* Clarification about cultures:
Direct set cultures do not always change the acidity of the milk. When cheese makers used to make their own cultures, they were much quicker to raise the acidity in the milk which meant there were more calcium ions. In the not-so-distant past, cheese makers made their own "bulk-set" cultures by inoculating sterilized milk with bacteria and then letting it set until the pH was low (4.6) and the calcium ion levels were high. When these bulk-set starters were used, the coagulation time was short and the curds were firm. (If you want to understand the exact chemical process, Paul Kinstedt explains it in American Farmstead Cheese, p. 90.)
This is not to be confused with making a mother culture from a direct-set culture. We explain this process in our book Home Cheesemaking, but we don't recommend it because the possibility for contamination is high and because it will be very difficult to achieve any consistency. (That said, if you are doing this, there is good troubleshooting info in The Cheesemaker's Manual, p. 46-48.) In summary – if you are using one of our cultures to make a mother culture, you will still need to use calcium chloride for all the reasons we mentioned previously.
If you are making your own cultures (from kefir grains, etc.) as in David Asher's The Art of Natural Cheesemaking, the same benefits from the bulk-set cultures will apply and you will most likely not need to use calcium chloride to raise the acidity. Asher does not condone the use of calcium chloride, even in goat's milk. He contends that if goats have "plenty of browse," their curds will be strong without it. (However, we have to have to point out that there may still be issues with the initial level of acidity in your milk if your goats are ill or in the late stages of lactation.)
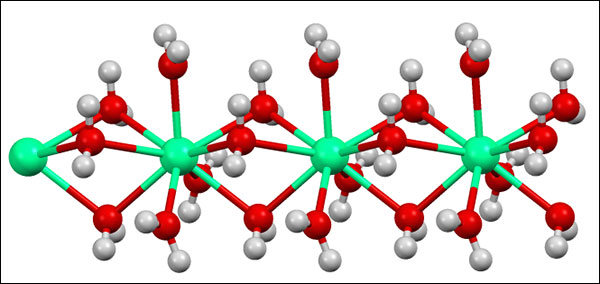
Structure of a diluted calcium chloride molecule. From Wikipedia: "Structure of the polymeric [Ca(H2O)6]2+ center in hydrated calcium chloride, illustrating the high coordination number typical for calcium complexes." If you know what this means, please share it with us in the comments!
Do I Need Calcium Chloride to Make Cheese
Source: https://blog.cheesemaking.com/calcium-chloride/